OMOP CDM
(Common Data Model)
OMOP/OHDSI services
Transform Healthcare Data & Real-World Data approach with OMOP/OHDSI Services

Global Healthcare Data: High heterogeneity & complexity
On an international scale, healthcare data are extremely heterogeneous, making their cross-disciplinary exploitation particularly complex, and sometimes even impossible.
- Different Data Sources : Clinical trials, Registries, Hospital data…
- Various Data Collection Methods : Data management system, questionnaire, case report form….
- International Data : Local regulations, local technology, native language…
- Multiple Healthcare Systems : EMR, private insurance, public claims…
OMOP CDM, a common model for health data
To overcome these challenges, OMOP CDM (Observational Medical Outcomes Partnership – Common Data Model) was launched in 2008: this private-public partnership between the FDA and several pharmaceutical companies aims to standardize the various sources of health observation data using a common data model.
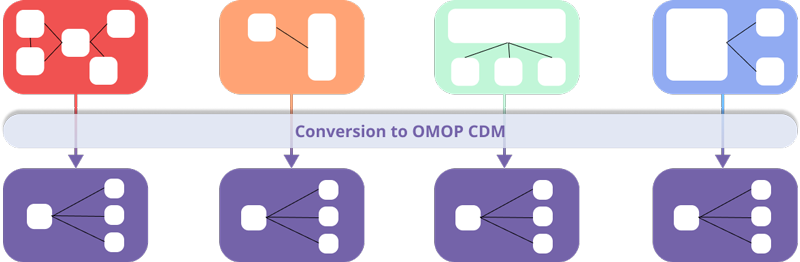
The OMOP CDM is a relational model for healthcare databases, designed to ensure interoperability between them.
The OMOP CDM allows the mapping of data sources to this standard format and the use of a unique terminology and collaborative tools.
Quinten Health is certified by EDHEN for standardization of health data to the OMOP Common Data Model and installation of analytical tools.
The EDHEN network leverages the OMOP Common Data Model to standardize health data across Europe, enhancing research and healthcare. By involving over 100 SMEs, it fosters interoperability and large-scale analysis, aiming to improve patient outcomes through data-driven insights.
5 advantages of standardizing your data into OMOP CDM
Facilicated collaboration among organizations & countries
A single data structure to enhance collaboration between organizations in different countries…
…And in a single common data “language” that binds them together.
Large-scale availability of structured
187 Data sources converted to OMOP format…
…in 29 european countries using the EHDEN network.
*As of November 2023 – source : www.ehden.eu
Better reproducibility of research studies
Studies are protocol-driven and thus 100% reproducible (on identical or different data)…
…to enable repeatable, generalizable, and calibrated research.
Greater efficiency in research activities
OMOP saves precious time in setting up and running analyses, thanks to the availability of open-source tools…
…and in generating evidence, thus considerably reducing the associated costs.
Robustness of Real-World Evidence Generation
OMOP ensures consistency and accuracy of data capture, which improves study reliability.
And, conducting larger-scale studies on various databases increases the quality of the generated evidence.
WE OFFER END-TO-END OMOP AND OHDSI CAPABILITIES
Entrust us with your projects and we’ll help you with our solutions
Conversion to OMOP
OMOP CDM Mapping
We provide complete solutions for converting and structuring your existing databases in line with the OMOP-CDM, facilitating their analysis, validation, and deployment.
OMOP data analysis
Advanced analytics, modeling and simulation
We have a team of experts skilled in the use of OHDSI packages and in the development of tools adapted to your needs.
Quinten Health, a pioneer in Real-World medical simulation
We work closely with regulators, payers, and leading healthcare and research institutions to help healthcare manufacturers develop better products faster and at lower cost, ultimately improving health for all.
A unique expertise in Real-World data science, predictive disease modeling, machine learning and statistical modeling, proven over the past decade.
An international network of opinion leaders, medical experts, regulatory authorities, and health technology assessment agencies.
A highly skilled team of over 50+ Real-World data science specialists, biostatisticians, epidemiologists, and biomedical experts.
More than 400+ completed projects in various therapeutic areas: oncology, rare diseases, endocrinology, cardiology, neurology, immunology, infectious diseases, gastroenterology.
Click here to download our booklet on OMOP/OHDSI


