More-Europa Project

More Effective and ethical Use of Registry data to suppOrt PAtient–centered regulatory and health technology assessment decision-making (MORE-EUROPA)
About More-EUROPA
The More-EUROPA project aims to enhance the ethical and efficient utilization of registry data to assist drug regulators and Health Technology Assessment (HTA) agencies in making patient-centered decisions. This initiative is financed by the Horizon Europe framework programme, which is the primary research and innovation fund in the European Union. Fourteen organizations, both public and private, from seven EU countries (including EMSP), will participate in the project, which will commence on January 1st, 2023, and run for a duration of five years.
Objectives of the More-EUROPA project
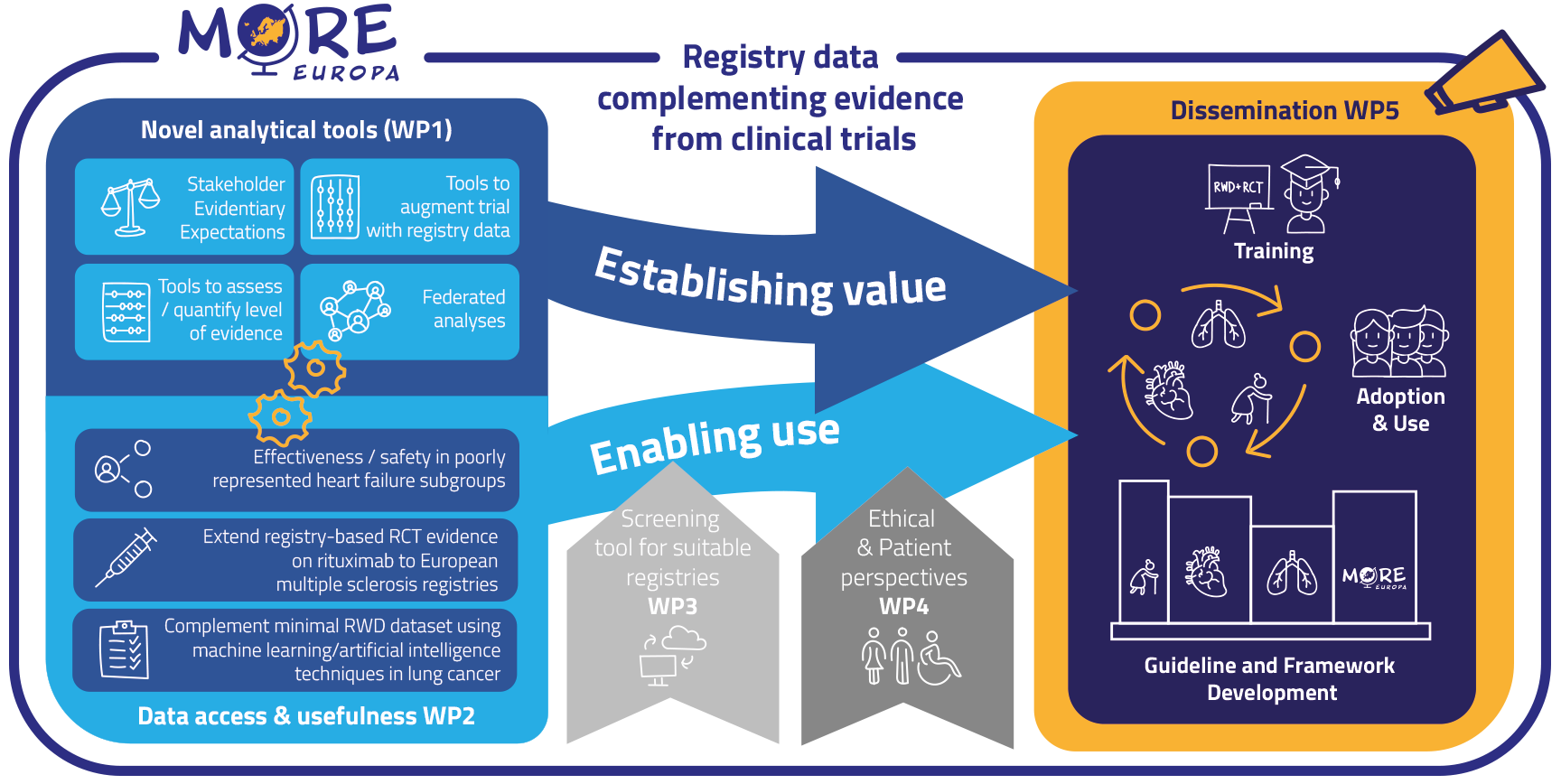
To achieve its ambitions, More-EUROPA has the following specific objectives:
1) Expand knowledge on drug efficacy and safety using RWD augmenting RCT data (establish value) using specific case studies in WP2 that are supported by novel analytical tools developed in WP1, and by creating a protocol for a registry-based RCT in WP5;
2) Develop a methodological framework (establish value) including analytical tools to integrate evidence derived from RCTs and (multiple) RWD sources (WP1);
3) Develop and standardize methods to increase usability of RWD across different registries (enable use) (WP1, WP2);
4) Develop a screening tool to timely identify suitable registries and RWD (enable use; WP3);
5) Develop an ethical framework describing practice-oriented ethical requirements (enable use) for generating and using patient-relevant RWD to support decision-making (WP4);
6) Create an integrated More-EUROPA framework, incorporating methodological tools and ethical considerations, to favour adoption and use of RWE across the drug lifecycle in regulatory & HTA guidelines and decision-making (WP5);
7) Increase the skills and competencies of HTA staff and regulators towards the use of RWD (favour adoption and use) through interactive multi-stakeholder training sessions (WP6);
8) Create an integrated multi-stakeholder platform for RWD/RWE engaging and aligning with other European.
Quinten Health’s contributions:
In this project, each work package will have a designated leader responsible for organizing the activities and communicating with relevant stakeholders. Quinten Health works on WP1, WP5, and WP6, and has taken the lead on WP3, which is centered on create a screening tool that can efficiently and effectively identify appropriate registries and Real-World Data (RWD) in a timely manner.
As an expert company in artificial intelligence using Real World Data, Quinten Health will support the following project’s objectives:
→ Expand the knowledge of drug efficacy and safety using Real World Data and to supplement RCT’s data using specific ‘case’ studies supported by developed new analytical tools;
→ Develop a methodological framework including analytical tools to integrate evidence derived from RCTs and (multiple) RWD sources;
→ Develop an ethical framework describing practice-based ethical requirements for generating and using patient-relevant RWD to support decision making;
→ Create an integrated framework, incorporating methodological tools and ethical considerations, to promote the acceptance and use of evidence based on RWD (=RWE, Real World Evidence) throughout the drug life cycle in drug registration and HTA guidelines and decision-making.
MORE-EUROPA INSIGHTS
Enhancing Patient-Centered Decision-Making: Quinte...
In efforts to push for the use of registry data to support patient-centered decision-making in regulatory and health technology assessment (HTA) pr...
Heading to ISPOR 2023, a deepdive in health econom...
Quinten Health joins ISPOR Europe 2023, the leading European conference for health economics and outcomes research (HEOR) in Copenhagen.We're...
Horizon Europe: Quinten Health into More-EUROPA...
Quinten Health is participating in the More-EUROPA project in the framework of the Horizon Europe program. The latter funds projects aiming to...