Connecting Electronic Health Records to a Biomedical Knowledge Graph to Link Clinical Phenotypes and Molecular Endotypes in Atopic Dermatitis
Publisher: Nature Scientific Reports Authors: Francesca Frau, Paul Loustalot, Margaux Törnqvist, Nina Temam, Jean Cupe, Martin Montmerle, Franck Augé View Publication Abstract Precision medicine is defined by the U.S. Food & Drug Administration as “an innovative approach to tailoring disease prevention and treatment that considers differences in people’s genes, environments, and lifestyles”. To succeed in […]
On the Concepts, Methods, and Use of “Probability of Success” for Drug Development Decision-Making: A Scoping Review
Publisher: Clinical Pharmacology and Therapeutics Authors: Aysun Cetinyurek Yavuz, Muhammad Bergas Nur Fayyad, Ce Jiang, Florie Brion Bouvier, Celine Beji, Sonia Zebachi, Ghinwa Y. Hayek, Billy Amzal, Raphael Porcher, Julien Tanniou, Kit Roes, Laura Rodwell View publication Abstract Drug development is a lengthy process with considerable uncertainty at each milestone. Several trials are needed to progress […]
A text-to-tabular approach to generate synthetic patient data using LLMs
Publisher: arXivAuthors: Margaux Tornqvist, Jean-Daniel Zucker, Tristan Fauvel, Nicolas Lambert, Mathilde Berthelot, Antoine Movschin View publication Abstract Access to large-scale high-quality healthcare databases is key to accelerate medical research and make insightful discoveries about diseases. However, access to such data is often limited by patient privacy concerns, data sharing restrictions and high costs. To overcome […]
Identification of risk profiles for liver injury in adults with multiple sclerosis using artificial intelligence method
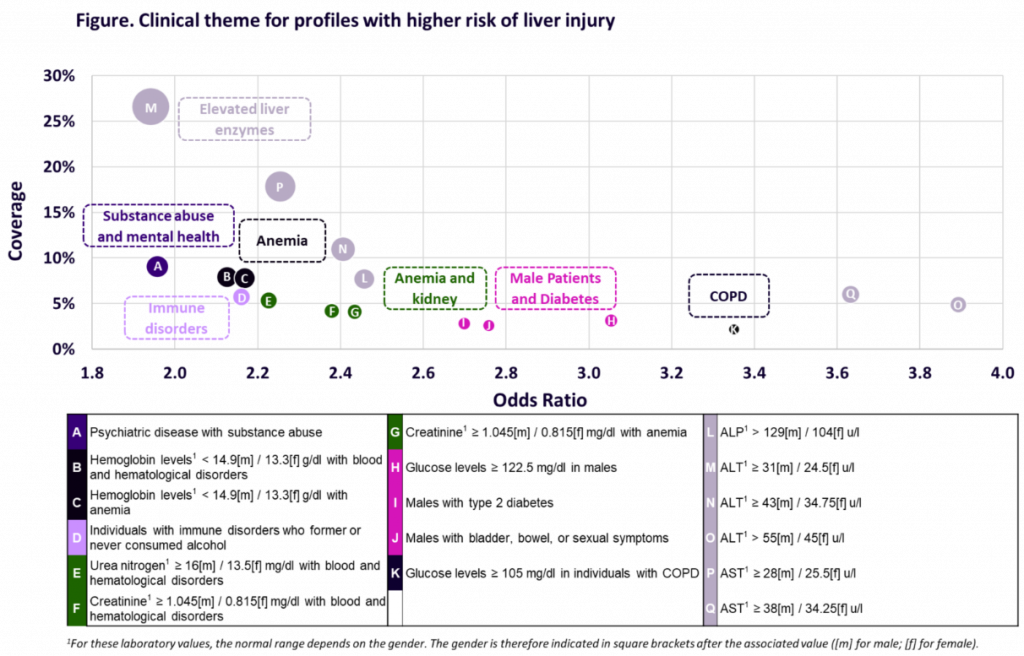
Event: The Liver Meeting – AASLD (American Association for the Study of Liver Diseases) 2024, San Diego, California, USA Authors: Dominique Larrey, Fang Liz Zhou, Claire Brulle-Wohlhueter, Myriam Benamor, Jeffrey Chavin, Neda Razaz, Raphael Bejuit, Julien Dauriat, Théophile Reppelin, Romane Péan, Sam Ekhtiari, Nicolas Wagner, Fred Lublin View Poster Background The evolution of multiple sclerosis (MS) […]
Identification of risk profiles for liver injury in adults with multiple sclerosis using artificial intelligence method
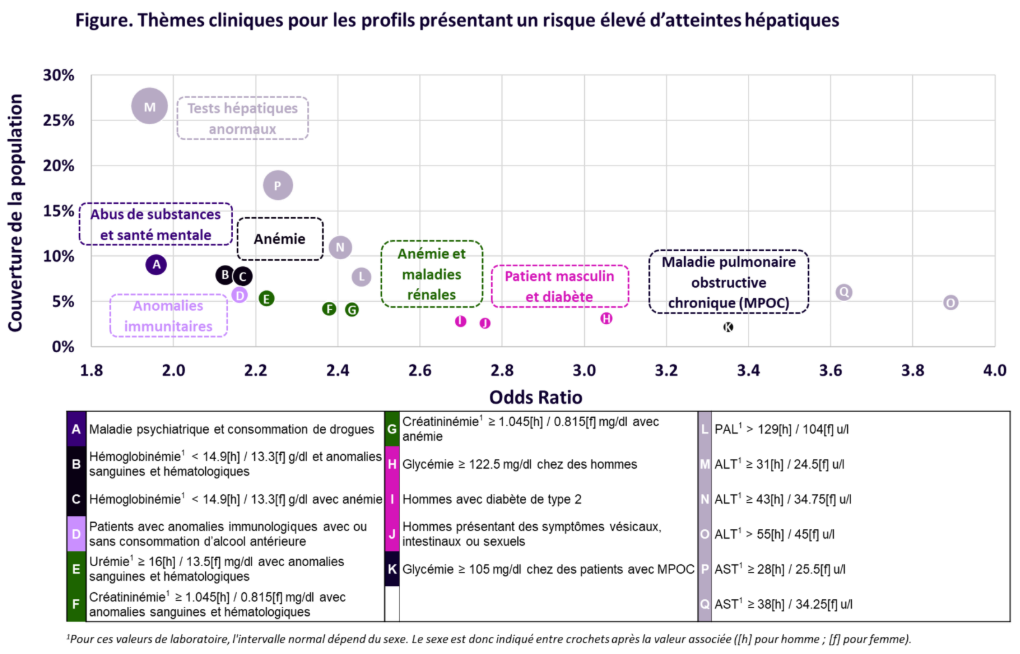
Authors: Dominique Larrey, Fang Liz Zhou, Claire Brulle-Wohlhueter, Myriam Benamor, Jeffrey Chavin, Neda Razaz, Raphael Bejuit, Julien Dauriat, Théophile Reppelin, Romane Péan, Sam Ekhtiari, Nicolas Wagner, Fred Lublin Event: Congrès AFEF (Association Française pour l’Étude du Foie) View Poster Introduction et Objectif L’évolution du traitement de la sclérose en plaques (SEP) au cours des deux dernières […]
Analyzing Recurrent Events in Multiple Sclerosis: A Review of Statistical Models with Application to MSOAC Trial

Event: ECTRIMS 2024, Copenhagen, Denmark Authors: D. Herman, J. Tanniou, N. Lambert, P. Loustalot, Q. Pilard View Poster Introduction In Multiple Sclerosis (MS), patients are likely to face repeated event such as confirmed disability progression (CDP). However, clinical trials often focus on analyzing time to first events, ignoring subsequent events, which are of clinical importance, despite the availability of statistical […]
Tailored diagnostic decision tree resulting from machine learning to improve early diagnosis of ASMD

Event: WORLDSymposium 2024 San Diego, USA Authors: M. Domenica Cappellini, R. Giugliani, M. Törnqvist, P. Guilmin, C.Clémente, M. Montmerle, A. Chiorean, T. Reppelin, Stefaan Sansen, Alexandra Dumitriu, Neha Shah, Maja Gasparic View Poster Abstract Acid sphingomyelinase deficiency (ASMD) is a rare and debilitating lysosomal storage disease and delays in diagnosis are common. We employed machine learning (ML) on electronic health […]
Existing practices for the identification, selection or assessment of registries for regulatory/HTA purposes: a More-EUROPA project systematic review

Event: ISPOR Europe 2023, Copenhagen, Denmark Authors: S. Zebachi, J. Moreira, J. Tanniou, P. Godbillot, P. Tang, B. Amzal View Poster Objectives Real-world data (RWD), particularly patient registries, represent a potential useful source of information and may be required at various stages of the drug lifecycle. Different questions may be asked throughout the regulatory and/or […]
What are unmet needs in the use of registries in regulatory/HTA decision-making in Europe? A survey-based approach from the More-EUROPA project

Event: ISPOR Europe 2023, Copenhagen, Denmark Authors: J. Moreira, J. Tanniou, P. Tang, P. Godbillot, F. Ahrens, B. Amzal View Poster Objectives The More-EUROPA project, involving 14 public and private organizations from 7 EU countries, evaluates the effective and ethical use of registry data to support patient-centered decisions by drug regulators and Health Technology Assessment (HTA) agencies in Europe. Specifically, one […]
Criteria and methods applied to decision-making to embark on late-stage clinical trials with a multi-stakeholder perspective

Event: ISPOR Europe 2023, Copenhagen, Denmark Authors: C. Jiang, J. Tanniou, B. Amzal View Poster Objectives The More-EUROPA project aims at establishing the value of registry-based RWD in enhancing RCT data and enabling a more effective and ethical use of registry data to support patient-centred regulatory and HTA decision-making. Work package 1 (WP1) is specifically […]

