Quinten Health presented at ISPOR Europe 2025 a new regulatory-grade chronic disease cohort simulator built from the CONSTANCES cohort linked and individually matched to the French SNDS.
This unique data asset provides an unprecedented foundation for generating transparent, reproducible, and high-quality real-world evidence for HTA, regulatory assessment, and long-term disease modelling.
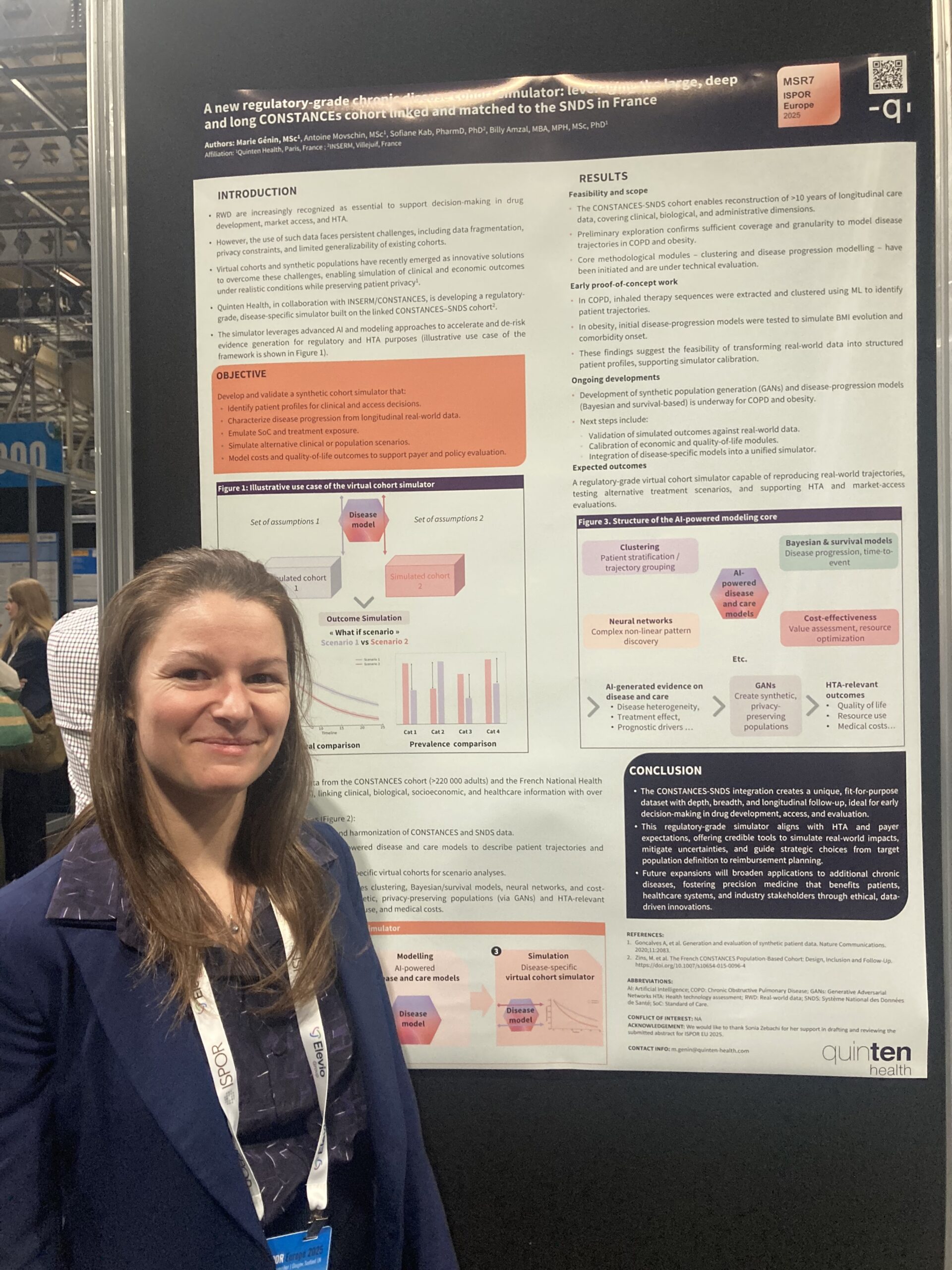
A Regulatory-Grade Resource Built from CONSTANCES and SNDS
The CONSTANCES cohort is a large, population-based, longitudinal French cohort containing detailed clinical, sociodemographic, lifestyle, and biological data. When linked to the comprehensive SNDS claims database, it creates a powerful environment for studying real-world patient trajectories, multimorbidity, healthcare utilization, and disease progression at scale.
The cohort simulator showcased at ISPOR leverages this combined dataset to support robust evidence generation across a wide range of chronic conditions.
Its depth and representativeness make it suitable for regulatory-grade modelling, including natural history reconstruction, long-term prediction, extrapolation studies, and comparative analyses required for HTA submissions.
Multi-Analytics Approach for Robust and Regulatory-Grade Modelling
A central component of the simulator is its multi-analytics framework, which integrates complementary methodological approaches to capture the complexity of chronic disease dynamics. Instead of relying on a single modelling paradigm, the simulator combines statistical modelling, machine learning, causal inference techniques, and health-economic methods to explore patient trajectories and outcomes from multiple angles.
This layered analytical strategy enables:
- robust disease-progression modelling, using both traditional longitudinal methods and modern AI architectures
- patient stratification and phenotype discovery, via clustering, dimensionality reduction, and trajectory-based methods
- causal and counterfactual analyses, supporting comparative effectiveness and “what-if” scenarios
- health-economic and quality-of-life modelling, integrated directly into disease simulations
- cross-validation of insights across multiple analytical lenses, strengthening confidence in regulatory-grade outputs
By integrating findings across these diverse analytical techniques, the simulator ensures methodological transparency, reproducibility, and resilience to modelling assumptions – key requirements for HTA bodies and regulatory stakeholders.
Supporting Reproducible and Trustworthy Evidence Generation
The simulator is designed to address a major challenge for stakeholders: producing transparent and reproducible real-world evidence that meets regulatory and HTA expectations.
Because it relies on a validated, population-based dataset, the simulator strengthens the credibility of:
- estimates of disease burden and trajectories,
- real-world treatment patterns and care pathways,
- risk factor evolution and their long-term impact,
- clinical outcomes in diverse patient populations.
It also supports the development of calibrated and validated chronic disease models, including sensitivity analyses and scenario exploration.
Applications for HTA Bodies, Pharmaceutical Companies, and Research Partners
The regulatory-grade simulator provides value for a wide range of decision-makers and evidence users:
For HTA bodies
- natural history reconstruction,
- robust and transparent parameters for economic models,
- real-world comparators and risk stratification.
For pharmaceutical companies
- long-term projections for therapeutic areas,
- real-world evidence supporting market access,
- treatment sequencing and patient pathway modelling.
For research partners
- epidemiological studies,
- disease burden assessments,
- development of advanced modelling and simulation methods.
Its versatility makes it a central asset for evaluating chronic diseases in real-world settings using a rich, validated French cohort.
Conclusion
This work, presented at ISPOR Europe 2025, illustrates how a well-structured and linked real-world data ecosystem can meaningfully support HTA and regulatory decision-making.
Leveraging the CONSTANCES cohort matched to the SNDS enables the creation of reliable, scalable, and transparent chronic disease models, ultimately strengthening the evidence used across healthcare evaluation and access pathways.
Official Poster
This poster was presented by Marie Génin at ISPOR Europe 2025. You can view the official poster page here:
ISPOR Europe 2025 – Official Poster Link


