Event: The Liver Meeting – AASLD (American Association for the Study of Liver Diseases) 2024, San Diego, California, USA
Authors: Dominique Larrey, Fang Liz Zhou, Claire Brulle-Wohlhueter, Myriam Benamor, Jeffrey Chavin, Neda Razaz, Raphael Bejuit, Julien Dauriat, Théophile Reppelin, Romane Péan, Sam Ekhtiari, Nicolas Wagner, Fred Lublin
Background
The evolution of multiple sclerosis (MS) treatment in the past two decades has raised concerns regarding potential liver injury (LI) associated with disease-modifying therapies (DMTs). Lifestyle and generic factors may also contribute to LI. Nevertheless, predicting and identifying risk factors for LI among MS patients remained challenging. This study aims to identify high-risk profiles for liver injury among MS patients by analysis of electronic health record (EHR) and the use of an artificial intelligence (AI) based subgroup discovery algorithm.
Methods
This retrospective cohort study was conducted using Optum Market Clarity EHR data. Adult patients with first known diagnosis of MS between January 1st, 2015, and December 31st, 2019 (cohort entry date (CED) was the date of the initial MS diagnosis) and actively managed in EHR during the baseline period (730 days prior to CED) were included in the study. Patients were excluded if they had a LI event or ALT>2xULN or a diagnosis of liver disease in the baseline period. Patients were followed until first LI event or 3 years after the CED whichever occurred earlier. LI event was defined either as meeting the international lab criteria (ALT, ALP, total bilirubin) or the presence of an acute LI diagnosis [Fontana et al., Drug Safety 2009]. Q-Finder, a supervised non-parametric proprietary subgroup discovery algorithm, was used to identify subgroups associated with increased LI risk and defined by baseline demographic and clinical characteristics. .
Results
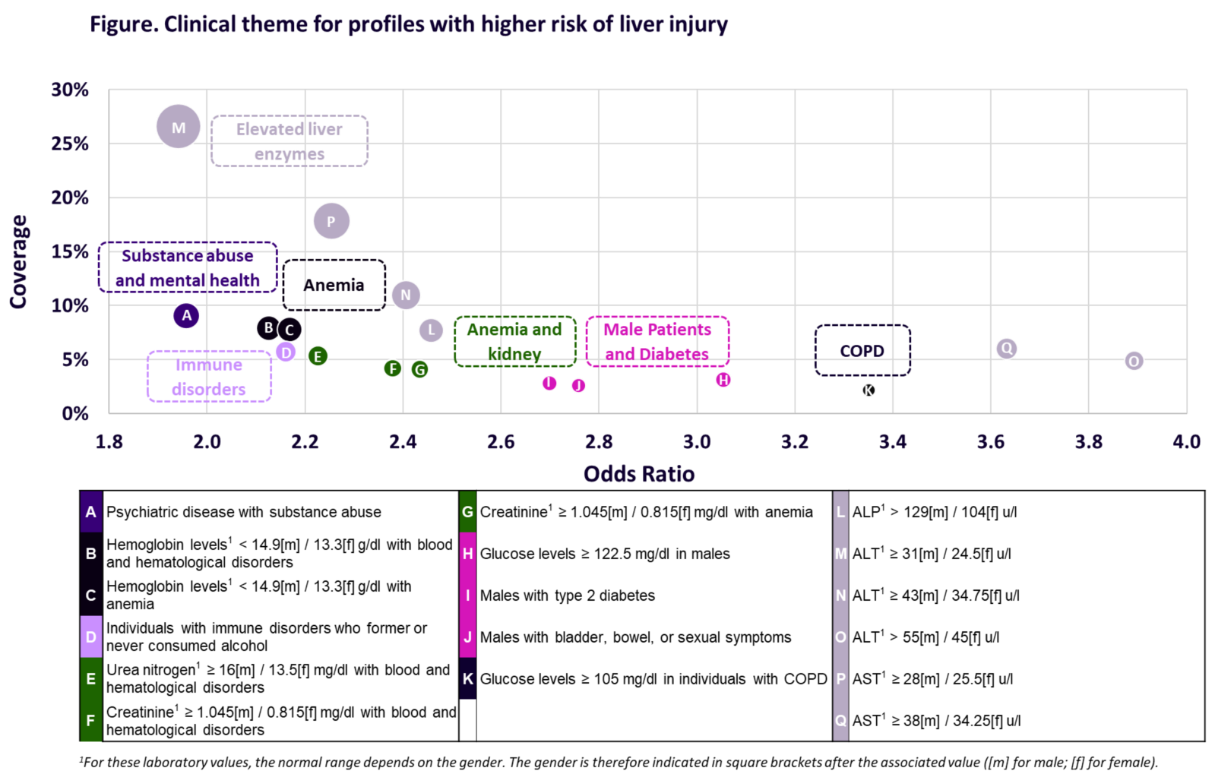
The analysis involved 5,986 MS patients, of whom 233 (4%) developed a LI event within 3 years following CED. The Q-Finder algorithm identified 6 risk factors associated with elevated levels of baseline liver enzymes (ALP, ALT, AST). Additionally, 11 clinically relevant risk factors were identified focusing on factors unrelated to liver enzymes, which were summarized into 6 clinical themes: substance abuse and mental health disorders, anemia, anemia and kidney, immune disorders, male patients and diabetes, and chronic obstructive pulmonary disease (COPD) (see Figure).
Conclusion
This study demonstrated that elevated liver enzymes at baseline were a strong risk driver for liver injury among MS patients. The Q-Finder also allowed the discovery of new risk factors that may help predict MS patients who are at higher risk of LI and provided insights to improve patient screening criteria for clinical trials and clinical management of MS.
Disclosure: Study funded by Sanofi